撰文:阴知远,康家辉,霍姝佳,徐海伟
视网膜神经元受损和丢失是视觉系统神经退行性疾病的特征,可能造成多种致盲性眼病[1, 2]。利用外源性和内源性干细胞实现向视网膜神经元分化是治疗神经退行性疾病的有效疗法[3]。然而,供体细胞的来源、安全性和有效性等问题限制了外源性干细胞的应用。以Müller胶质(Müller glia,MG)细胞为代表的内源性干细胞在低等脊椎动物中表现出非凡的再生能力[4],然而,哺乳动物中Müller胶质细胞失去了重编程能力。分析Müller胶质细胞重编程能力的物种差异性,探索Müller胶质细胞重编程的策略并寻找更先进的重编程技术,对于提高哺乳动物中Müller胶质细胞的重编程能力具有重要的意义。
陆军军医大学第一附属医院徐海伟团队在【中国神经再生研究(英文版)】(Neural Regeneration Research)2025年第4期发表的综述文章总结更新了过去十年来在小鼠中成功地进行Müller胶质细胞重编程策略的最新进展,讨论了与Müller胶质细胞重编程相关的挑战,并结合神经系统再生的研究进展,提出了六种有前景的小鼠Müller胶质细胞重编程策略,包括表观遗传重塑、代谢调节、免疫调节、化学小分子诱导、细胞外基质重塑和细胞融合,为开发小鼠Müller胶质细胞重编程的新策略提供了理论基础。
神经胶质细胞在特定条件下可通过增殖重编程为视网膜神经元。在低等脊椎动物,如斑马鱼中,当视网膜受到急性损伤后,Müller胶质细胞可去分化为神经胶质来源的祖细胞,继而转分化为视网膜神经元,修复整个视网膜。而小鼠和人类视网膜中的Müller胶质细胞很少表现出重编程为视网膜神经元的倾向。比较Müller胶质细胞对视网膜损伤反应的种属差异性[5, 6],研究视网膜细胞发育[7]以及体细胞向神经元的重编程[8]为探索小鼠Müller胶质细胞重编程开辟了新的可能性。
目前,研究人员已经开发出十余种促进小鼠Müller胶质细胞重编程为视网膜神经元的策略[9-18]。但目前的Müller胶质细胞重编程策略仍存在一些问题和争议。如:重编程的效率和有效性较低,Müller胶质细胞来源的视网膜神经元与视网膜宿主神经元的差异性,在复杂的损伤环境中神经元存活及视功能改善水平的不确定性[18-21]。面临着遗传因素、表观遗传因素和复杂微环境等多种限制,迫切需要探索更先进的重编程策略。
Müller胶质间接重编程和直接重编程
该综述将小鼠体内Müller胶质细胞重编程的方法分为「间接重编程」和「直接重编程」两种。其中,「间接重编程」是将成体细胞诱导、去分化为多能干细胞,再分化成特定的细胞类型[5]。「直接重编程」是将成体细胞直接转分化为其他细胞类型,不经历去分化阶段[22](图1)。
在斑马鱼中,视网膜受损后,Müller胶质细胞被凋亡细胞激活,进入「间接重编程」状态。部分Müller胶质细胞去分化获得了干细胞的特征,然后转分化为视网膜神经元,新生的神经元整合到现有的神经元回路,修复损伤的视网膜[5]。间接重编程需要获得稳定的多能性和重置表观遗传特征[23]。Müller胶质细胞的间接重编程不会导致Müller胶质细胞的短缺,因而更有利于视网膜的修复。但仍需进一步提高Müller胶质细胞去分化、增殖和转分化操作的精确性。而将小鼠Müller胶质细胞直接重编程为视网膜神经元是一个更快、更高效的过程,且保留了与年龄相关的表观遗传特征,在组织修复方面具有独特的优势[23]。Müller胶质细胞的直接和间接重编程策略都存在争议和难度,目前的重编程策略并没有完全实现细胞类别的转化,迫切需要新的Müller胶质细胞重编程策略。
目前,Müller胶质细胞重编程的策略主要是通过调控Müller胶质细胞中特定的基因,特别是转录因子来实现的。然而,目前筛选出来的用于调节小鼠Müller胶质细胞重编程的转录因子相对较少,通过利用多组学技术[14] 或采用基于算法的转录因子预测和全基因组详尽的CRISPR激活来筛选作用于细胞命运转化的转录因子和其他DNA结合调节因子,可以提高筛选调节小鼠Müller胶质细胞重编程的基因的效率[24]。
诱导小鼠Müller胶质细胞重编程的潜力策略
开发新的策略是实现小鼠Müller胶质细胞重编程的另一种选择。多种非Müller胶质细胞靶向的调节方法可用于Müller胶质细胞重编程的实现,如表观遗传重构、代谢调节、免疫调节、化学小分子调控、细胞外基质重构和细胞-细胞融合(图2)。
表观遗传重构 Müller胶质细胞重编程的遗传学,特别是对谱系特异性转录因子的研究取得了相当大的成就。Ascl1和NeuroD1、Otx2、Crx、Nrl、Pou4f2、Atoh7和Islet1是不同视网膜细胞发育的关键转录因子[25],被广泛用于小鼠的Müller胶质细胞重编程。DNA甲基化、DNA乙酰化、组蛋白或非组蛋白修饰、染色质重塑和非编码RNA调控等表观遗传重构方式,极大地影响了细胞命运的决定[26-28],对Müller胶质细胞重编程至关重要,是促进小鼠Müller胶质细胞重编程的一种有前景的方法。
免疫调节 小胶质细胞是视网膜固有的巨噬细胞,视网膜受损时会产生一系列的炎症反应,导致小胶质细胞的激活。在斑马鱼中,急性炎症激活视网膜中的小胶质细胞,启动并促进Müller胶质细胞的重编程[29]。而哺乳动物中,小胶质细胞在视网膜损伤和退行性病变期间持续过度激活,引起慢性炎症,加速视网膜感光细胞凋亡,Müller胶质细胞细胞增殖[30]。小胶质细胞对Müller胶质细胞重编程的影响表现出物种多样性,改变小胶质细胞表型将是促进Müller胶质细胞重编程的有效策略。未来的研究需要进一步区分小胶质细胞表型的差异,理解小胶质细胞和Müller胶质细胞之间的交互作用,以便于通过间接地、精细地调节小胶质细胞的表型来促进小鼠Müller胶质细胞重编程。
Müller胶质细胞重编程过程中的重要信号通路包括JAK-STAT信号通路[31, 32]、NF-κB信号通路[33, 34]、mTOR信号通路[35]和TNF-α[36]等,均与细胞因子介导的免疫过程相关。未来需要进行全面的研究来确定急性和慢性炎症调节的靶点在哺乳动物视网膜再生中的潜在作用。
代谢调节 代谢的变化可以决定干细胞的命运和功能 [37, 38]。消除代谢障碍可提高直接神经元重编程的效率[39]。多种成体细胞在向干细胞和神经元重编程的过程中伴有糖酵解[40]、氧化磷酸化[41]和脂质过氧化的变化[39]。单独或联合使用抗氧化剂和其他药物调节Müller胶质细胞代谢是实现Müller胶质细胞重编程的可行方法。
线粒体的结构和活性对细胞重编程也有显著影响[42-44]。线粒体移植不仅具有神经保护特性,而且能使自体视网膜干细胞转分化为视网膜神经节细胞再生[45-47]。细胞外囊泡可以调节Müller胶质细胞的代谢微环境,通过移植外泌体有望促进Müller胶质细胞重编程[48]。虽然目前还没有通过线粒体移植和EV移植实现Müller胶质细胞重编程的成功案例,但干细胞和癌细胞代谢重编程的研究为小鼠Müller胶质细胞重编程提供了许多启示,是未来可研究的方向之一。
化学小分子诱导 小分子具有细胞渗透性,可以直接靶向细胞内和细胞表面蛋白,在细胞信号转导和表观遗传修饰中发挥重要作用[49]。化学小分子注射不仅可通过直接重编程方式改变体细胞类别[50-52],还可以诱导小鼠和人体细胞进入多能干细胞[53-55]。与基因组编辑技术相比,小化学分子不会整合到基因组,高度可控和高效,易于优化、标准化、制造,可联合使用[49]。因此,使用小化学分子Müller胶质细胞重编程是细胞命运操纵的理想策略。然而,血-视网膜屏障是小分子进入视网膜的主要障碍。在开发小分子药物时,必须考虑到脱靶效应的风险[21]。
细胞外基质重塑 眼睛的细胞外基质(Extracellular matrix,ECM)包括基底膜和非基底膜基质,前者包括内界膜和Bruch膜,后者包括光感受器外节周围的光感受器间基质、填充视网膜下腔以及连接视网膜细胞的突触周围的突触基质[56]。Müller胶质细胞是ECM分泌的主要来源。在疾病、损伤、视网膜去分化和再生的背景下,ECM伴随着广泛的重构过程。来自ECM的机械信号在将体细胞重编程为干细胞中发挥着重要作用,但这一过程的机制尚未明确[57]。大多数ECM分子促进神经胶质瘢痕的形成,并创建一个抑制视网膜再生的环境[58]。再殖的小胶质细胞可能通过调节ECM重塑而促进Müller胶质细胞获得祖细胞样特征[59]。视网膜细胞周围的微环境影响Müller胶质细胞重编程过程,但对其参与程度还需要进一步的探索。
细胞-细胞融合 近年来,一种基于视网膜细胞间相互作用的新型Müller胶质细胞非靶向调控方法已经出现。细胞-细胞融合可以引起Müller胶质细胞内基因表达的实质性变化,重置多能干基因的表达,并完全逆转体细胞类别的谱系[60]。将造血干细胞或骨髓细胞募集到小鼠视网膜中,可驱动Müller胶质细胞分别向光感受器祖细胞或神经节和无长突细胞重编程 [9, 10]。这些研究表明,细胞-细胞相互作用是实现Müller胶质细胞重编程的一种创新方法。
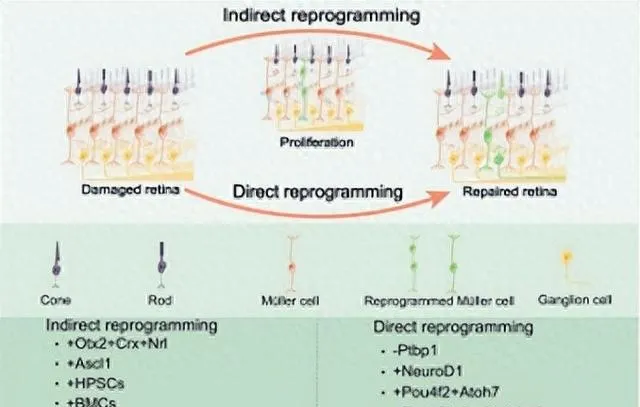
图1 间接重编程和直接重编程示意图
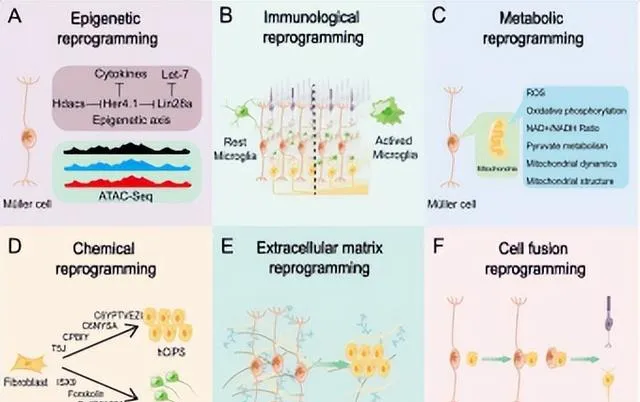
图2 六种诱导小鼠Müller胶质细胞重编程的新策略
在过去的十年中,成年小鼠Müller胶质细胞重编程策略和机制的研究发展令人鼓舞。到目前为止,在小鼠中已经实现了相对成功的Müller胶质细胞重编程。为了进一步开发新的Müller胶质细胞重编程策略,作者们除了提供筛选识别更多的转录因子的方法,还提出了六种有前景的小鼠Müller胶质细胞重编程策略。联合使用多种策略可能是未来Müller胶质细胞重编程策略的发展趋势。
然而,实现小鼠Müller胶质细胞的重编程仍存在诸多挑战。比如如何提高重编程的效率和稳定性、将Müller胶质细胞来源的神经元视网膜回路整合,采用何种有效的工具来追踪Müller胶质细胞细胞在体内的命运。此外,基于工具创建者、建模者和实验者之间的成功合作,将对神经胶质生物学的发展至关重要,这可能会引发Müller胶质细胞重编程研究中的重大进展。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01612
参考文献
[1] Cheng YJ, Lin CH, Lane HY. From menopause to neurodegeneration-molecular basis and potential therapy. Int J Mol Sci. 2021;22:8654.
[2] Wan J,Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev. 2016;40:41-47.
[3] Ikelle L, Al-Ubaidi MR, Naash MI. Pluripotent stem cells for the treatment of retinal degeneration: current strategies and future directions. Front Cell Dev Biol. 2020; 8:743.
[4] Jin ZB, Gao ML, Deng WL, et al. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019;69:38-56.
[5] Lahne M, Nagashima M, Hyde DR, et al. Reprogramming Müller glia to regenerate retinal neurons. Annu Rev Vis Sci. 2020; 15:171-193.
[6] Hamon A, Roger JE, Yang XJ, et al. Müller Glial cell-dependent regeneration of the neural retina an overview across vertebrate model systems. Dev Dyn. 2016; 245:727-738.
[7] Buono L, Martinez-Morales JR. Retina development in vertebrates: systems biology approaches to understanding genetic programs: on the contribution of next-generation sequencing methods to the characterization of the regulatory networks controlling vertebrate eye development. Bioessays. 2020;42:e1900187.
[8] Gascon S, Masserdotti G, Russo GL, et al. Direct neuronal reprogramming: achievements, hurdles, and new roads to success. Cell Stem Cell. 2017;21:18-34.
[9] Sanges D, Simonte G, Di Vicino U, et al. Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors. J Clin Invest. 2016;126:3104-3116.
[10] Pesaresi M, Bonilla-Pons SA, Simonte G, et al. Endogenous mobilization of bone-marrow cells into the murine retina induces fusion-mediated reprogramming of müller glia cells. EBioMedicine. 2018;30:38-51.
[11] Jorstad NL, Wilken MS, Grimes WN, et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103-107.
[12] Jorstad NL, Wilken MS, Todd L, et al. STAT signaling modifies Ascl1 chromatin binding and limits neural regeneration from Müller glia in adult mouse retina. Cell Rep. 2020;30:2195-2208.e2195.
[13] Yao K, Qiu S, Wang YV, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 2018; 560:484-488.
[14] Hoang T, Wang J, Boyd P, et al. Gene regulatory networks controlling vertebrate retinal regeneration. Science. 2020;370:eabb8598.
[15] Zhou H, Su J, Hu X, et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell. 2020; 181:590-603. 603.
[16] Xiao D, Jin K, Qiu S, et al. In vivo regeneration of ganglion cells for vision restoration in mammalian retinas. Front Cell Dev Biol. 2021;9:755544.
[17] Todd L, Jenkins W, Finkbeiner C, et al. Reprogramming Müller glia to regenerate ganglion-like cells in adult mouse retina with developmental transcription factors. Sci Adv. 2022;8:eabq7219.
[18] Pinsonneault CBP, David LA, Fernandes JAL, et al. Direct neuronal reprogramming by temporal identity factors. Proc Natl Acad Sci U S A. 2023; 120:e2122168120.
[19] Hoang T, Kim DW, Appel H, et al. Ptbp1 deletion does not induce astrocyte-to-neuron conversion. Nature. 2023; 618:E1-7.
[20] Xie Y, Zhou J, Wang LL, et al. New AAV tools fail to detect Neurod1-mediated neuronal conversion of Müller glia and astrocytes in vivo. EBioMedicine. 2023; 90:104531.
[21] Zhang H, Guo Y, Yang Y, et al. MAP4Ks inhibition promotes retinal neuron regeneration from Müller glia in adult mice. NPJ Regen Med. 2023; 8:36.
[22] Wang H, Yang Y, Liu J, et al. Direct cell reprogramming: approaches, mechanisms and progress. Nat Rev Mol Cell Biol. 2021; 22:410-424.
[23] Samoylova EM, Baklaushev VP. Cell reprogramming preserving epigenetic age: advantages and limitations. Biochemistry (Mosc). 2020; 85:1035-1047.
[24] Liu Y, Yu C, Daley TP, et al. CRISPR activation screens systematically identify factors that drive neuronal fate and reprogramming. Cell Stem Cell. 2018;23:758-771.e758.
[25] Xiang M. Intrinsic control of mammalian retinogenesis. Cell Mol Life Sci. 2013;70:2519-2532.
[26] Mitra S, Sharma P, Kaur S, et al. Histone deacetylase-mediated Müller glia reprogramming through Her4.1-Lin28a axis is essential for retina regeneration in zebrafish. iScience. 2018;7:68-84.
[27] Aldiri I, Xu B, Wang L, et al. The dynamic epigenetic landscape of the retina during development, reprogramming, and tumorigenesis. Neuron. 2017;94:550-568.e510.
[28] Xie X, Fu Y and Liu J. Epigenetic regulation of somatic cell reprogramming. Curr Opin Genet Dev. 2017;46:104-113.
[29] Iribarne M. Inflammation induces zebrafish regeneration. Neural Regen Res. 2021;16:1693-1701.
[30] Wang M, Ma W, Zhao L, et al. Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011; 8:173.
[31] Gorsuch RA and Hyde DR. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014;123:131-140.
[32] Wan J, Zhao XF, Vojtek A, et al. Retinal injury, growth factors, and cytokines converge on beta-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014;9:285-297.
[33] Palazzo I, Deistler K, Hoang TV, et al. NF-kappaB signaling regulates the formation of proliferating Müller glia-derived progenitor cells in the avian retina. Development. 2020;147:dev183418.
[34] Palazzo I, Todd LJ, Hoang TV, et al. NFkB-signaling promotes glial reactivity and suppresses Müller glia-mediated neuron regeneration in the mammalian retina. Glia. 2022;70:1380-1401.
[35] Zhang Z, Hou H, Yu S, et al. Inflammation-induced mammalian target of rapamycin signaling is essential for retina regeneration. Glia. 2020;68:111-127.
[36] Nguyen-Chi M, Laplace-Builhé B, Travnickova J, et al. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death & Disease. 2017; 8:e2979-e2979.
[37] Zheng X, Boyer L, Jin M, et al. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. elife. 2016;10:e13374.
[38] Cliff TS and Dalton S. Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Curr Opin Genet Dev. 2017;46:44-49.
[39] Gascon S, Murenu E, Masserdotti G, et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016; 18:396-409.
[40] Tsogtbaatar E, Landin C, Minter-Dykhouse K, et al. Energy metabolism regulates stem cell pluripotency. Front Cell Dev Biol. 2020; 8:87.
[41] Magistretti PJ and Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883-901.
[42] Polyzos AA, Lee DY, Datta R, et al. Metabolic reprogramming in astrocytes distinguishes region-specific neuronal susceptibility in Huntington mice. Cell Metab. 2019; 29:1258-1273 e1211.
[43] Bahat A, Gross A. Mitochondrial plasticity in cell fate regulation. J Biol Chem. 2019;294:13852-13863.
[44] Liu W, Long Q, Chen K, et al. Mitochondrial metabolism transition cooperates with nuclear reprogramming during induced pluripotent stem cell generation. Biochem Biophys Res Commun. 2013;431:767-771.
[45] Nascimento-Dos-Santos G, de-Souza-Ferreira E, Lani R, et al. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. Biochim Biophys Acta Mol Basis Dis. 2020; 1866:165686.
[46] Wu SF, Lin CY, Tsai RK, et al. Mitochondrial transplantation moderately ameliorates retinal degeneration in royal college of surgeons rats. Biomedicines. 2022; 10:2883.
[47] Zhang J, Wu S, Jin ZB, et al. Stem cell-based regeneration and restoration for retinal ganglion cell: recent advancements and current challenges. Biomolecules. 2021; 11:987.
[48] Fridman ES, Ginini L, Gil Z. The role of extracellular vesicles in metabolic reprogramming of the tumor microenvironment. Cells. 2022;11:1433.
[49] Wang J, Sun S and Deng H. Chemical reprogramming for cell fate manipulation: Methods, applications, and perspectives. Cell Stem Cell. 2023; 30:1130-1147.
[50] Xia X, Teotia P, Patel H, et al. Chemical induction of neurogenic properties in mammalian Müller glia. Stem Cells. 2021;39:1081-1090.
[51] Zhang L, Yin JC, Yeh H, et al. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell. 2015; 17:735-747.
[52] Mahato B, Kaya KD, Fan Y, et al. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature. 2020; 581:83-88.
[53] Hou P, Li Y, Zhang X, et al. Pluripotent stem cells inducedfrom mouse somatic cells by small-molecule compounds. Science. 2013; 341:4.
[54] Liuyang S, Wang G, Wang Y, et al. Highly efficient and rapid generation of human pluripotent stem cells by chemical reprogramming. Cell Stem Cell. 2023; 30:450-459 e459.
[55] Guan J, Wang G, Wang J, et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. 2022; 605:325-331.
[56] Serjanov D and Hyde DR. Extracellular matrix: the unexplored aspects of retinal pathologies and regeneration. Adv Exp Med Biol. 2023; 1415:309-317.
[57] Liu L, Liu M, Xie D, et al. Role of the extracellular matrix and YAP/TAZ in cell reprogramming. Differentiation; research in biological diversity. 2021; 122:1-6.
[58] Reinhard J, Joachim SC, Faissner A. Extracellular matrix remodeling during retinal development. Exp Eye Res. 2015;133:132-140.
[59] Cheng X, Gao H, Tao Z, et al. Repopulated retinal microglia promote Müller glia reprogramming and preserve visual function in retinal degenerative mice. Theranostics. 2023; 13:1698-1715.
[60] Brown KE and Fisher AG. Reprogramming lineage identity through cell-cell fusion. Curr Opin Genet Dev. 2021; 70:15-23.
文章摘要: 视网膜神经元受损和丢失是视觉系统神经退行性疾病的特征,可能造成多种致盲性眼病。利用外源性和内源性干细胞实现向视网膜神经元分化是治疗神经退行性疾病的有效疗法。然而,供体细胞的来源、安全性和有效性等问题限制了外源性干细胞的应用。以Müller胶质细胞为代表的内源性干细胞在低等脊椎动物中表现出非凡的再生能力,然而,哺乳动物中Müller胶质细胞失去了重编程能力。分析Müller胶质细胞重编程能力的物种差异性,探索Müller胶质细胞重编程的策略并寻找更先进的重编程技术,对于提高哺乳动物中Müller胶质细胞的重编程能力具有重要的意义。文章总结更新了过去十年来在小鼠中成功地进行Müller胶质细胞重编程策略的最新进展,讨论了与Müller胶质细胞重编程相关的挑战,并结合神经系统再生的研究进展,提出了六种有前景的小鼠Müller胶质细胞重编程策略,包括表观遗传重塑、代谢调节、免疫调节、化学小分子诱导、细胞外基质重塑和细胞融合,为开发小鼠Müller胶质细胞重编程的新策略提供了理论基础。
文章来源: Yin Z, Kang J, Cheng X, Gao H, Huo S, Xu H (2025) Investigating Müller glia reprogramming in mice: a retrospective of the last decade, and a look to the future. Neural Regen Res 20(4):946-959.











